Background: Observation or "wait and watch" (W/W) strategy remains a viable option in the rituximab era for asymptomatic, stage II-IV, low-tumor burden patients with FL grade 1-2, 3A. Studies to date both in pre and post rituximab era have not shown an overall survival benefit from immediate treatment in such low-risk patients.To improve our understanding and to better counsel FL patients on W/W strategy, we sought to estimate the incidence of treatment initiation at landmark time points in our prospectively observed cohort from the Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE). We further evaluate the association between the presence of GELF criteria (MER treatment criteria) at diagnosis and initiation of treatment patterns, transformation rates and cause of death in FL patients managed by W/W.
Methods: FL patients on W/W strategy were identified from MER of the University of Iowa/Mayo Clinic Lymphoma SPORE. From 2002-2015, consecutive patients with newly diagnosed FL were offered enrollment. Patients were managed per treating physician and followed prospectively. Baseline clinical and pathological data were abstracted using a standard protocol. Cumulative incidence estimates of treatment initiation for follicular lymphoma were calculated using transformation to large cell lymphoma (as the first event) and death due to any cause as competing risks. Transformation was defined based on biopsy-proven disease. Patients were retrospectively considered to meet treatment criteria at diagnosis if they had the presence of any GELF criteria components per available abstracted data in the MER database. Not all GELF criteria were prospectively assessed and captured for all patients, and thus the MER treatment criteria utilized here will be conservative for formal GELF assessment.
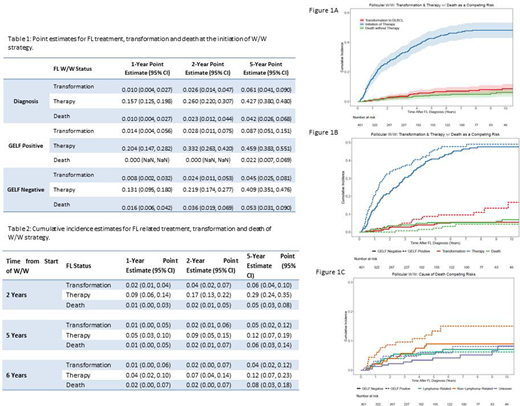
Results: A total of 401 FL patients were identified in MER on W/W strategy. Baseline characteristics for W/W patients showed a favorable profile such as normal LDH (89%), low and intermediate FLIPI score in 48% and 35% respectively, no B symptoms (97%), low tumor burden (<4 nodal groups) in 72%, and GELF positive vs. negative in 36% and 64% respectively. At a median follow up of 8 years (IQR 5.9-12), 64% initiated treatment for FL only. Cumulative incidence estimates of treatment initiation at sequential time points were calculated from the initiation of observation (Table1&2). At diagnosis, the likelihood of treatment initiation was 15%, 26%, and 42% in the next 1, 2, and 5 years. The incidence of treatment initiation decreased over time as patients remained treatment-free after diagnosis (Figure 1A [blue curve]). Patients that met MER treatment criteria had increased rates of therapy initiation (33.2% vs. 21.9%) in the next two years and untreated transformation to large cell lymphoma [red curve] (8.7% vs. 4.5%) during the next 5 years (Figure 1B). Despite this, the rate of lymphoma related death at 10 years was similar between groups (met treatment criteria = 6.2% vs. 7.1%) (Figure 1C, [green curve]).
Discussion:
The longer duration of W/W strategy suggests a decreasing need for treatment over time. We provide time point estimates, which would be helpful for counseling patients. Of note, long-term continuous oncologic assessment and follow-up is necessary since approximately 66% of patients in this mature cohort initiated treatment by 8 years median follow up. MER treatment criteria at diagnosis identified patients with higher rates of transformation and therapy initiation in the first two years but did not identify those with worse lymphoma specific survival. Identification of biological differences in patients with early vs. late or no progression is a critical next step in understanding outcomes in W/W patients.
Ansell:Bristol Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Takeda: Research Funding; AI Therapeutics: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; ADC Therapeutics: Research Funding; Trillium: Research Funding. Cerhan:BMS/Celgene: Research Funding; NanoString: Research Funding. Wang:Incyte: Research Funding; Innocare: Research Funding; Novartis: Research Funding. Witzig:MorphSys: Consultancy; AbbVie: Consultancy; Incyte: Consultancy; Acerta: Research Funding; Karyopharm Therapeutics: Research Funding; Immune Design: Research Funding; Spectrum: Consultancy; Celgene: Consultancy, Research Funding. Maurer:Morphosys: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Nanostring: Research Funding; Kite: Membership on an entity's Board of Directors or advisory committees; Celgene / BMS: Research Funding. Nowakowski:NanoString: Research Funding; Kite: Consultancy; Seattle Genetics: Consultancy; Kymera: Consultancy; Celgene/BMS: Consultancy, Research Funding; MorphoSys: Consultancy, Research Funding; Curis: Consultancy; Ryvu: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal